



Colombia’s Health Regulatory Body Instituto Nacional de Vigilancia de Medicamentos y Alimentos – INVIMA has issued a health alert after uncovering a counterfeit batch of oncological drug MABTHERA® (500 mg/50 mL) circulating in the market.
The falsified product was marked as lot N7747 with a manufacture date of October 2023 and an expiry date of October 2026.
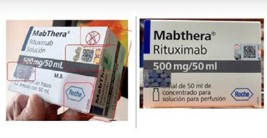
The finding was reported to the Institute by the holder of the health record, Hoffmann-La Roche., who confirmed that the said batch does not exist in any of the authorised presentations either in Colombia or in Mexico, which allowed for verification of its counterfeiting.
Invima warned that counterfeit medicines pose a direct risk to patients’ health, as they fail to meet quality, safety and efficacy standards.
Citizens were urged to purchase medicines only from authorised distributors and verify product authenticity via Invima’s official database.
The counterfeit pack was detected thanks to several visual discrepancies – the most telling being an incorrect silver holographic label. Invima noted that the dimensions, QR code, and reflective pattern of the fake hologram did not match those on genuine MABTHERA® packaging.
Other signs of forgery included cut edges on the labels, spelling mistakes, inconsistent typography, and barcode errors on the folding box.
These visual cues, combined with holographic inconsistencies, enabled experts to flag the counterfeit batch quickly.
According to William Saza Londoño, Coordinator of Invima’s Pharmacovigilance Group, the authentic MABTHERA® Concentrated Solution for Infusion (500 mg/50 mL) holds Health Registration No. 2010 MBT-0010348 and is distributed exclusively through Roche and its authorised importer.